The term ‘perovskite’ describes the general class of materials which are defined by an elemental composition ABX3 and show the crystal structure of the archetypical perovskite CaTiO3. These materials have applications in many fields, for example high temperature superconductors or batteries.
Recently, the class of organic-inorganic lead halide perovskites (A=CH3NH3, B=Pb, I=I,Br,Cl) has received great attention due to their application in photovoltaic devices and the spectacular rise in power conversion efficiencies, which are now exceeding 20% (http://www.nrel.gov/ncpv/images/efficiency_chart.jpg). The ground-breaking initial work on perovskite solar cells was driven by the Snaith group at Oxford University and research teams at EPFL (Switzerland) and KRICT (Korea). Our group has recently shown that these materials have great potential for the use in light emitting diodes, with bright emission over the visible range, and optically pumped lasing structures (LED and Lasing).
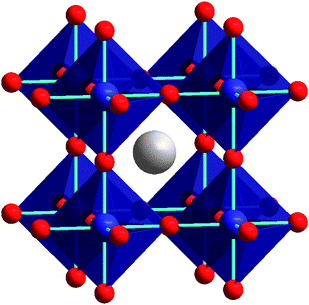
Recent reports have shown that the chemical versatility of these systems can be used to tune the optical bandgap of lead-halide perovskites over the visible range, i.e. by and changing the organic or halide part (Link to Adityas JPCL paper, J. Phys. Chem. Lett., 2014, 5 (15), pp 2501–2505, DOI: 10.1021/jz501332v). This color tuneability makes these materials particularly interesting for luminescent applications.
Despite the promising performance in devices, the fundamental photophysics of these materials are still under investigation. There is evidence for the intrinsic formation of free charge carriers in these materials which can show lifetimes in the order of 100s of nanoseconds (Link to JPCL paper, J. Phys. Chem. Lett., 2014, 5 (8), pp 1421–1426, DOI: 10.1021/jz5005285). However, the fundamental nature of these excited charger carriers, for example band structure and effective mass, is still under investigation. We are interested in the question how material composition changes the nature of the photoexcited states in these materials and which effects different levels of confinement have (Perovskite fast photophysics).
Image taken from P. Woodward et al., Cation ordering in perovskites, Journ. Mat. Chem. 20, 5785-96 (2010).
